Scoping review and evidence gap map on adverse events and health effects associated with plasma donation
06/11/23
Plasma-derived medicinal products (PDMPs) are essential for the prophylaxis and treatment of several disorders. They usually stem from source plasma that is obtained through plasmapheresis, a procedure whereby plasma (the liquid component of blood) is removed while blood cells are returned to the donor. The production of PDMPs currently mostly relies on plasma that is collected outside of Europe, with the United States of America (USA) as the main provider. A European dependency on the USA for source plasma entails a considerable risk of shortages, especially during health crises such as COVID-19.
In order for plasma collection to be upscaled in Europe without jeopardizing the health of plasma donors, evidence-based guidelines are needed. Regulations regarding the remuneration of donors and the maximum allowed donation frequency differ considerably between Europe and the USA. The European SUPPLY project (Strengthening voluntary non-remunerated plasma collection capacity in Europe) aims to prepare recommendations for safely increasing plasma collection in the European Union, in order to ensure a stable supply of PDMPs.
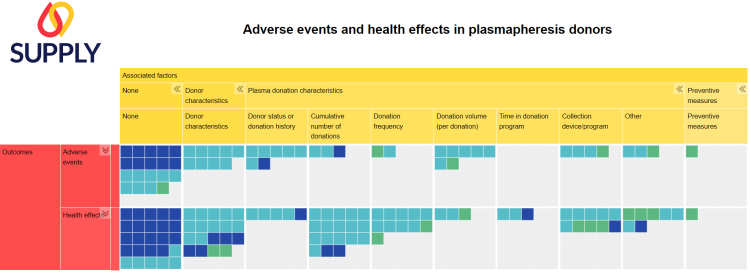
Within the project, we have published a scoping review aimed at systematically mapping the available evidence regarding adverse events and possible health effects in plasmapheresis donors. In addition, factors that were studied in association with such events/effects were identified, including the status of the donor (first-time or repeat), the cumulative number of donations, the donation frequency, and preventive measures. In October 2022, we searched six databases and three registries, and identified 94 research articles and five registrations. We synthesized study characteristics narratively as well as in an interactive evidence gap map. The scoping review itself has been published in Vox Sanguinis.
This scoping review will hopefully help researchers and policy-makers to efficiently identify the available evidence and existing research gaps regarding the safety of plasmapheresis. If Europe wants to increase plasma collection, it is of paramount importance to consider the effect of frequent and long-term plasmapheresis. We found that there is a need for more controlled prospective studies with long-term donor follow-up. Furthermore, additional experimental studies comparing the health effects of different donation frequencies are required in order to define a safe upper limit for plasma donation frequency.
